Five Phase 3 Trials Inspected:
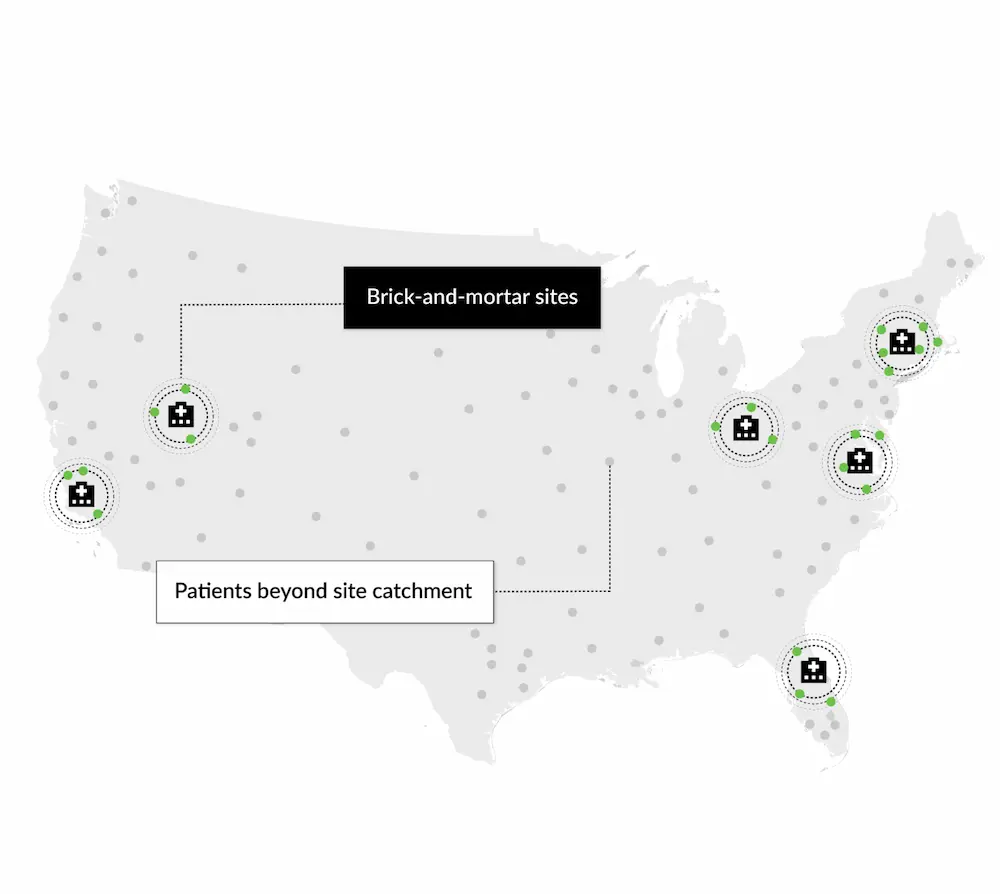
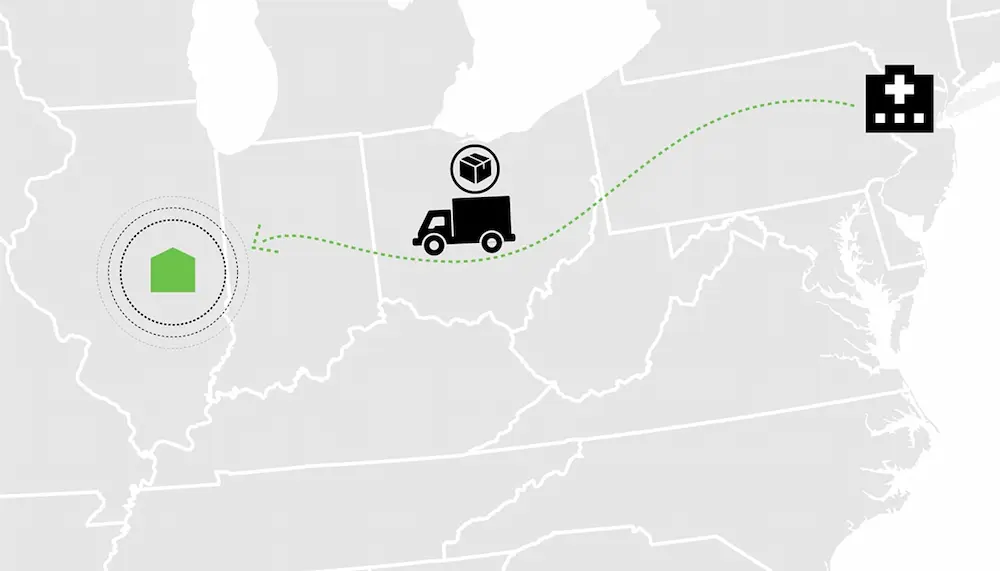
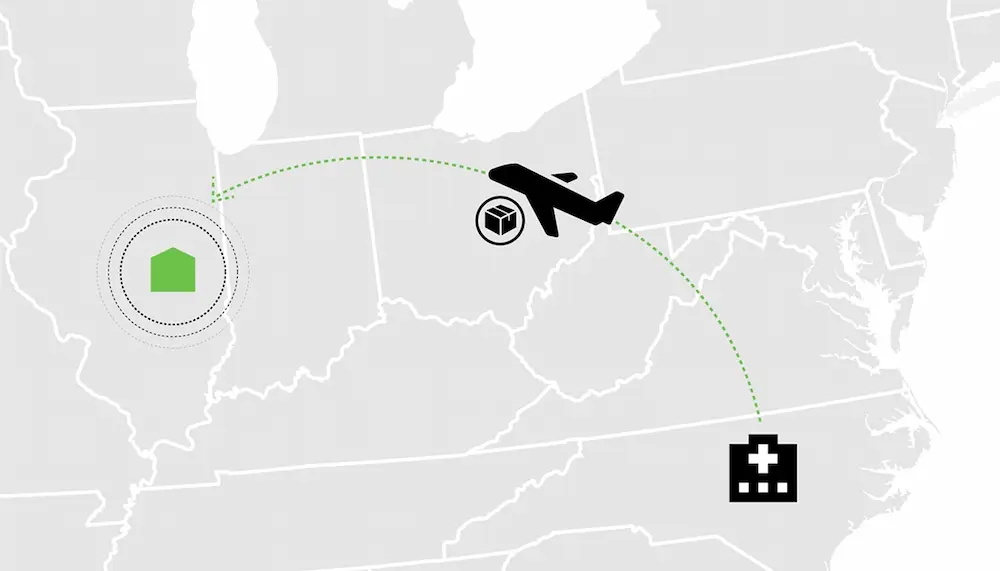
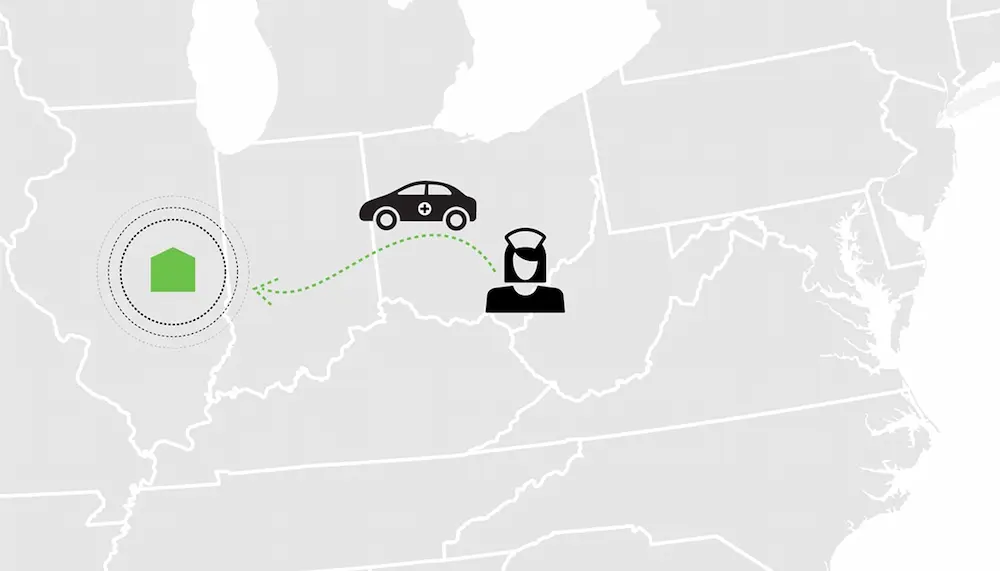
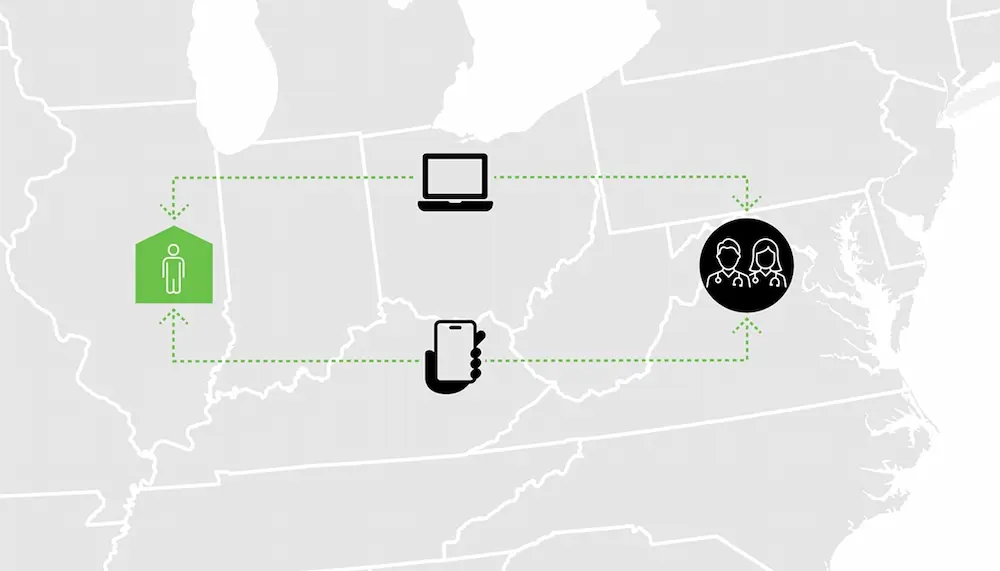


Acne Vulgaris
Alopecia
Atopic Dermatitis (Moderate to Severe)
Atopic Dermatitis (Face & Neck)
Basal Cell Carcinoma
Chronic Spontaneous Urticaria
Epidermolysis Bullosa
Herpes Labialis
Hidradenitis Suppurativa (HS)
Netherton Syndrome
Pemphigus Vulgaris
Psoriasis
Rosacea
Achondroplasia
Congenital Adrenal Hyperplasia
Chronic Kidney Disease
Diabetic Kidney Disease
Distal Renal Tubular Acidosis
Hypoparathyroidism
Nonalcoholic Fatty Liver Disease (NAFLD)
Phenylketonuria (PKU)
Prader-Willi Syndrome (PWS)
Severe Hypertriglyceridemia
T1D, T2D, and T3D Obesity
Celiac Disease
Cholestatic Pruritus
Cholestatic Pruritus Due to Primary Biliary Cholangitis (PBC) or Primary Sclerosing Cholangitis (PSC)
Congenital Sucrase-Isomaltase Deficiency (CSID)
Eosinophilic Esophagitis (EE)
Diabetic Gastroparesis
Primary Biliary Cholangitis (PBC)
Primary Sclerosing Cholangitis (PSC)
Short Bowel Syndrome and Intestinal Failure (SBS-IF)
Bacterial Infections
B-cell Mediated Autoimmune Disorders
C. Difficile Infection
Chronic Granulomatous Disease
Chronic Spontaneous Urticaria
COVID-19 & COVID-19 Vaccine
COVID-19 Prophylaxis
COVID-19/IC Prevention
Migraine
Herpes Labialis
Human Immunodeficiency Virus (HIV)
Hypogammaglobulinemia
Immunocompromised Patients
Mycobacterium Infections, Nontuberculous
Tuberous Sclerosis Complex (TSC)
Respiratory Viral Infections
Respiratory Syncytial Virus (RSV) Vaccine
Alzheimer's
Bipolar Depression
Cluster Headache
Epilepsy
GM2 Gangliosidosis
Huntington's Disease
Idiopathic Hypersomnia
Major Depressive Disorder (MDD)
Multiple Sclerosis (MS)
Narcolepsy
nOH in Multiple System Atrophy
Parkinson's Disease
Postpartum Depression
Rare Neurodevelopmental Disorder
Restless Leg Syndrome
Seizure
Age-Related Macular Degeneration
ALK+ non-NSCLC Tumors
Basal Cell Carcinoma
B-cell Chronic Lymphocytic Leukemia
B-cell Mediated Autoimmune Disorders
Benign Hematologic Disorders
Breast Cancer
Chronic Myeloid Leukemia
Colorectal Cancer (CRC)
Focal Segmental Glomerulosclerosis
Healthy Subjects for Cancer Screening
Lung Cancer
PBCAR-T Long-Term Follow-Up
Relapsed/Refractory Multiple Myeloma
Severe Hemophilia A
Solid Tumors
Sickle Cell Disease
Unspecified Cancer
Age-related Macular Degeneration
Wet AMD
Achondroplasia
Classical Homocystinuria (HCU)
Epidermolysis Bullosa
Fragile X Syndrome (FXS)
GM2 Gangliosidosis
Netherton Syndrome
Primary Biliary Cholangitis (PBC)
Primary Sclerosing Cholangitis (PSC)
Post-Allogeneic Hematopoietic Cell Transplant
Rare Neurodevelopmental Disorder
Severe Hemophilia A
Sickle Cell Disease
Spinal Muscular Atrophy
Vascular Ehlers-Danlos Syndrome (vEDS)
Von Willebrand Disease
22q11 Deletion Syndrome (Pediatric)
Asthma
Breathing Disorders
Chronic Obstructive Pulmonary Disease (COPD)
Chronic Rhinosinusitis w/ Nasal Polyps
Hypereosinophilic Syndrome (HES)
Idiopathic Pulmonary Fibrosis
Pulmonary/Respiratory Complications in Patients with Neuromuscular Diseases (NMDs)
Refractory Chronic Cough
Gout
Knee Osteoarthritis
Rheumatology Basket Study
Tophaceous Gout