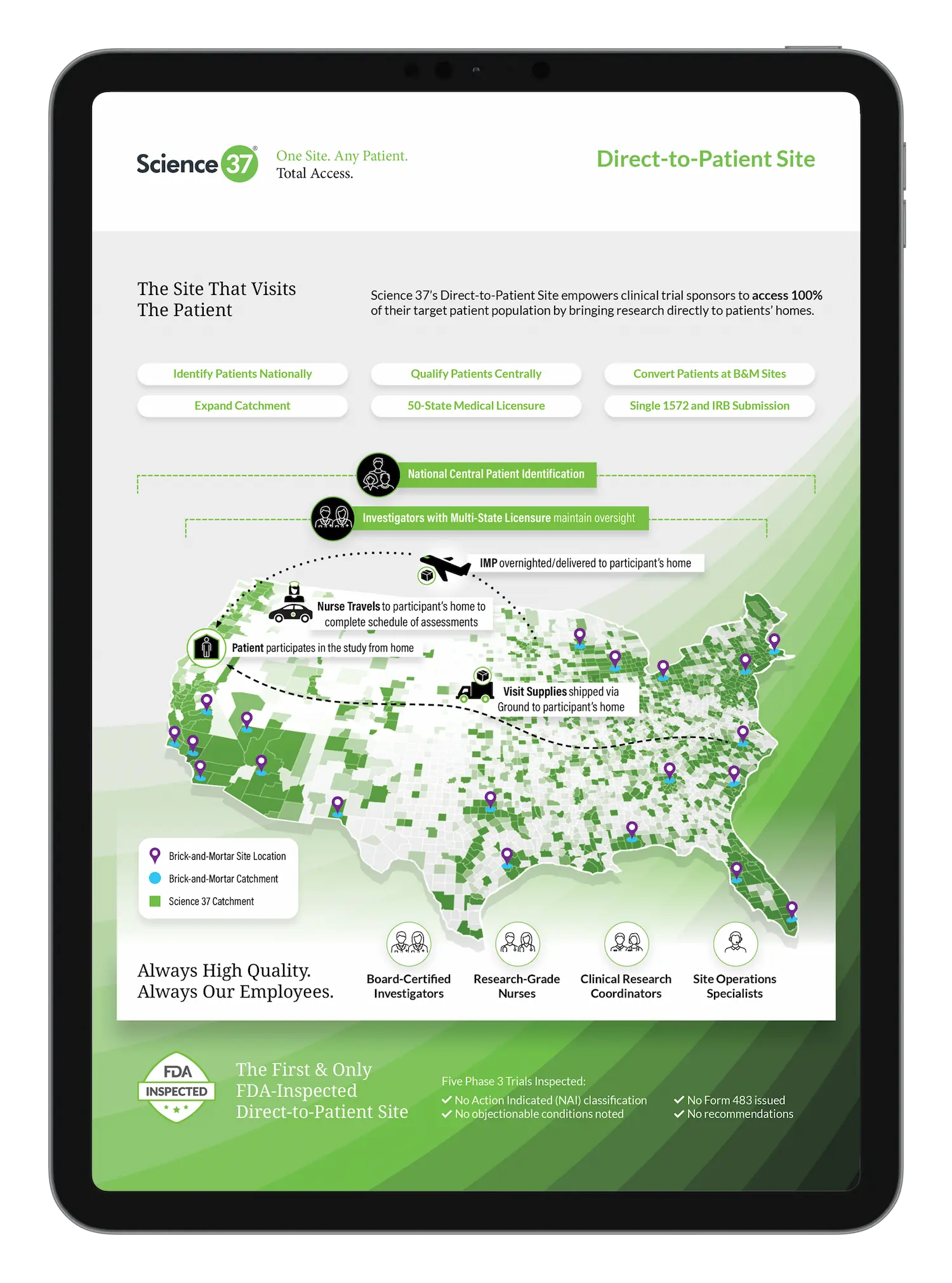
Scientific Poster

Preliminary Results from an Interventional Decentralized Clinical Trial
A top pharma company partnered with Science 37 to conduct the first-ever interventional decentralized clinical…