Science 37
How Science 37 Partners with Contract Research Organizations (CROs).
Science 37 provides CROs with a trusted site that visits the patient—without boundaries—to speed up recruitment expand diversity and extend patient access beyond traditional sites for clinical trials in any indication.

In-house Clinical and Operational Expertise
Science 37 delivers in-house clinical and operational expertise in remote clinical trial conduct across multiple therapeutic areas including oncology dermatology infectious diseases rare diseases and CNS disorders. A connected network of local labs community providers telemedicine investigators and mobile nurses provide care on patients' terms.
In-house Clinical and Operational Expertise
Science 37 delivers in-house clinical and operational expertise in remote clinical trial conduct across multiple therapeutic areas including oncology dermatology infectious diseases rare diseases and CNS disorders. A connected network of local labs community providers telemedicine investigators and mobile nurses provide care on patients' terms.

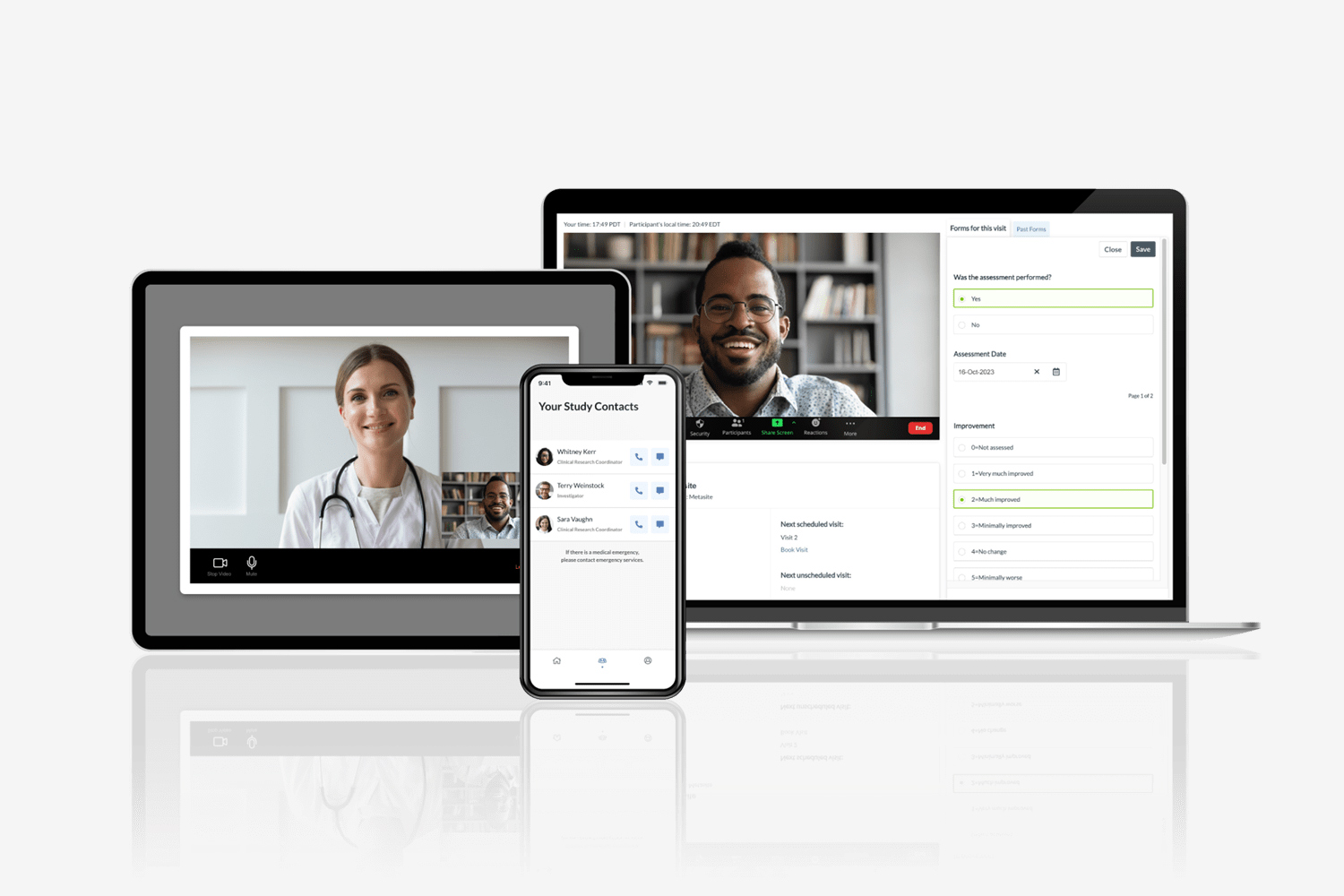
Integrated Data Management
Science 37 works with CROs to map out the entire clinical data journey to unify a single-source-of-truth in a central electronic data capture (EDC) system—regardless of how and where the data will be collected. Science 37’s technology integrates seamlessly with existing clinical trial management systems, eliminating the need for source data verification and enabling our partners to allocate valuable resources elsewhere.

Integrated Data Management
Science 37 works with CROs to map out the entire clinical data journey to unify a single-source-of-truth in a central electronic data capture (EDC) system—regardless of how and where the data will be collected. Science 37’s technology integrates seamlessly with existing clinical trial management systems, eliminating the need for source data verification and enabling our partners to allocate valuable resources elsewhere.
Regulatory Guidance at Every Step
Science 37’s regulatory experts lead the industry in navigating the disparate guidelines for decentralized clinical trial conduct. As no two studies are the same—and the regulatory framework around virtual trials is still evolving—Science 37 works with partners upfront on a regulatory strategy most appropriate for the therapeutic indication and the target market.