Blog
What Clinical Trial Patient Participants Want

Author: Darcy Forman, Chief Delivery Officer, Science 37
In clinical trials, participant satisfaction is critical to achieve the best outcomes in patient care and research goals. A new, groundbreaking research study conducted by Life Science Strategy Group reveals the perceptions and preferences of trial participants as well as the general public, and how those determinants impact all factors of the trial lifecycle.
These in-depth, granular results based on surveys with 360 clinical trial participants and 440 non-participants in both the United States and in Europe provides the most detailed and clearest look yet at what clinical trial factors are the most highly valued by patients — and which can inadvertently threaten a trial’s success by inhibiting recruitment, retention or longitudinal goals. Trial administrators and managers can see in empirical terms how a robust investment in flexible clinical trial infrastructure improves patient satisfaction and trial outcomes.
“Our research has identified those decentralized clinical trial elements that patients and non-participants value even more than direct interaction with a physician, and lead to greater satisfaction and willingness to participate in the future,” said Jon Meyer, Principal, Life Science Strategy Group.
The following takeaways from this landmark study crystallize the importance of agility and decentralization.
The “hassle factor” is a huge barrier to clinical trial enrollment
The likelihood — or even the perception — of inconvenience or an inflexible protocol is enough to deter patients. Trial administrators that struggle to recruit risk losing considerable time and money if they need to submit protocol amendments or initiate rescue study interventions.
These survey results are unique in that they quantify — across trial types, demographics and therapeutic areas — just how strong people’s aversion to inflexible clinical trial formats really is.
Consider this: Among survey respondents who have NOT participated in a clinical trial, roughly one in three said they were turned off by the prospect of inconvenience or travel difficulties. Collectively, “hassle factor” was a greater deterrent than concern about trial safety, which was cited by 28% as a reason for nonparticipation, or even not having a need to participate, which was cited by 26%.
By adhering to rigidly structured, site-based trial formats, trial administrators are turning off a plurality of potential subjects — a huge pool of people that otherwise might readily participate in a clinical trial.
An agile trial framework that allows for a variety of in-person, at-home and remote touch points will be able to streamline enrollment. The survey found that 56% of people who have participated in a clinical trial within the past two years did so because they found the requirements convenient or imposed little to no travel burden.
Travel can be a deterrent when not well-targeted
A trial format that is launched on a platform equipped to incorporate hybrid or remote elements facilitates more strategic use of travel. By replacing some in-person visits to a lab site or physician’s office with telehealth or mobile nursing care, trial coordinators can offer greater flexibility and autonomy to participants.
The survey revealed that reduction — although not elimination — of travel should be a key goal.
Among patients who reported having to travel at least a few times a week as a requirement of their clinical trial participation, 50% had a positive view of their in-person doctor visits. Half of the participants who traveled frequently for their trial also said that seeing their doctor in person left a favorable impression regarding their willingness to participate in future trials.
These findings imply that trial administrators can improve compliance and foster greater satisfaction by being strategic about the necessary use of in-office or in-lab visits that require participants to travel. Making a clinical trial easier for patients to accommodate increases the chances that they will stick with it for the full length of the study. This, in turn, improves both their own health outcomes as well as the data quality collected by the administering staff.
Unfamiliar technology lowers satisfaction and participation intent
Traditional site-based clinical research doesn’t leverage available technologies that could give patients more autonomy and greater choice around how, when and where they access care. Tech tools play a key role in the quest to improve the clinical trial experience, but too many manufacturers and CROs fail to use the right ones.
By and large, the technology that makes the most impactful difference for patients in their day-to-day lives is that which is familiar to them.
This study points to a high degree of comfort with and satisfaction with remote tools and comfort with familiar technology. The survey found that collecting health information via the patient’s own smart mobile device was ranked as being easier than via a wearable device by a significant margin — 77% versus 64%. By contrast, a mere 8% characterized using their smartphone or tablet as “difficult,” compared to 19% who said wearing a device was difficult.
When patients use technological devices and features with which they are already familiar, they report overwhelmingly positive impacts on their satisfaction and successful completion of the trial.
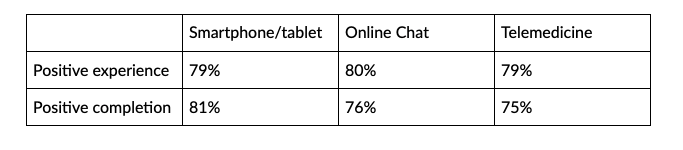
Accordingly, an interface that makes data capture hassle-free and lets patients receive care, ask questions and access medical advice using their own smartphones and tablets will elicit greater satisfaction and outcomes.
Recruitment and retention suffer without in-home care options
Manufacturers and CROs struggle to recruit enough patients because the traditional site-based model is perceived as inconvenient. When a trial administrator can offer flexible options such as in-home care, more people are willing to consider participating.
Nearly two in three trial participants prefer future trials to have more remote technology that facilitates in-home care, and among those, 29% “strongly prefer” trials that incorporate features like the use of smartphones and tablets, online patient portals, telemedicine, and in-home contact with care providers.
And among the general population — that is, survey respondents who had not participated in a clinical trial within the past two years — nearly two in three people expressed a preference for more in-home trial elements.
Patient retention, willingness and ability to complete a trial is hindered by a lack of flexibility inherent in site-based models, especially for trials with a high frequency of travel. Travel itself is not the problem, but frequent travel is a deterrent to completion and willingness to participate in future trials.
Incorporating decentralized elements can mitigate this by driving satisfaction with more flexible tools. Among trial participants who had to travel frequently, 100% reported positive associations with the use of online patient diaries, 80% characterized being able to email their doctor as “very positive” and 67% said the same about their interactions with in-home nursing providers.
Learn more about how decentralized clinical trials and a flexible, nimble technological platform can make trial operations simpler and less expensive by offering options for how care is delivered and data is collected. See how enhancing data-capturing capability can improve patient outcomes and yield superior results by clicking here.